

What trend in atomic radius occurs down a group on the periodic table? What causes this trend? Atomic radius increases going down a group because more energy levels are added. What trend in atomic radius occurs down a group on the periodic table what causes this trend? This is because, within a period or family of elements, all electrons are added to the same shell. Values are given for typical oxidation number and coordination. Covalent radius Half of the distance between two atoms within a single covalent bond. These values were determined using several different methods. Atomic size gradually decreases from left to right across a period of elements. Atomic radius, non-bonded Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. Ionization energy tends to increase across a period because electrons are added to the same main energy level Ionization energy tends to increase across a period because the nuclear charge increases.Ītomic radius patterns are observed throughout the periodic table. Why are the trends and exceptions to the trends in ionization energy observed? What is atomic radius of sodium?ĭoes Na or o have a larger ionization energy? Within a group, the ionization energy decreases as the size of the atom gets larger. This stronger attraction makes it more difficult to remove electrons. The more protons in the nucleus, the stronger the attraction of the nucleus to electrons. How does the trend in ionization differ from the trend in atomic size? So the nucleus has less of a effect on it’s electrons thus increasing the size of the atomic radius. What causes this trend? Atomic radius of elements tend to increase down a group because the shielding effect is overcoming the large nuclear force.

What trend in atomic radius occurs across the periodic table what causes this trend? Across a period, effective nuclear charge increases as electron shielding remains constant.
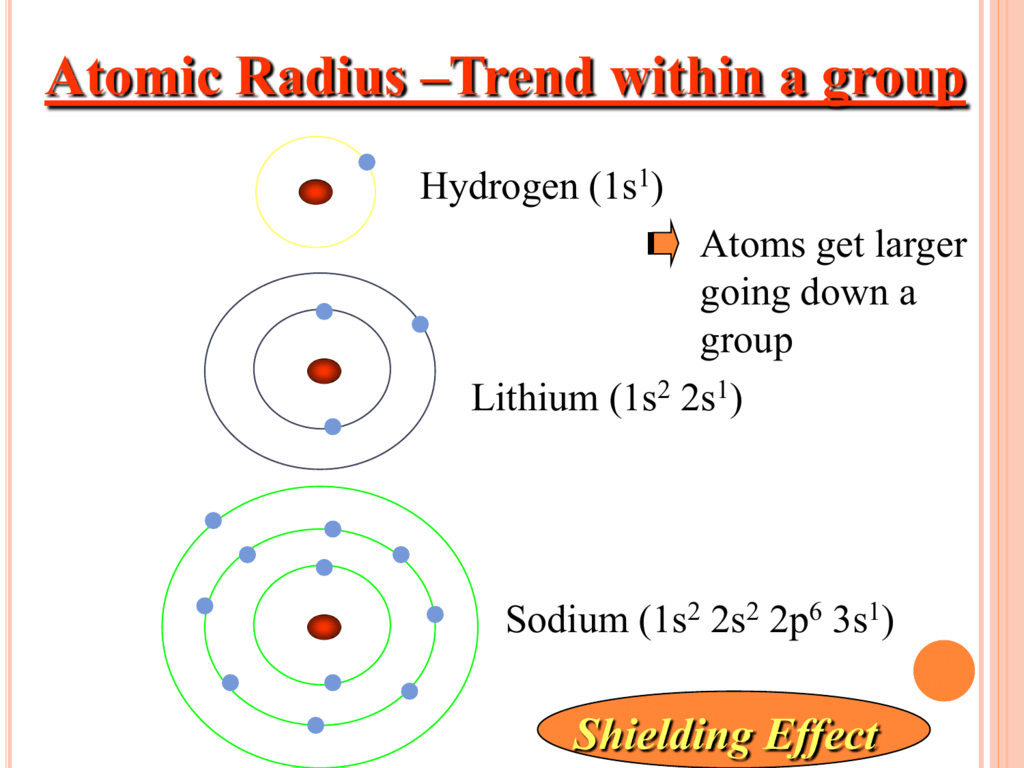
In general, atomic radius decreases across a period and increases down a group. Ionization energy increases from left to right across the periodic table.Ītomic radius is the distance from the atom’s nucleus to the outer edge of the electron cloud. Ionization energy decreases as we go down a group. Ionization energy refers to the amount of energy needed to remove an electron from an atom. What is the trend on the periodic table for ionization energy?


 0 kommentar(er)
0 kommentar(er)
